
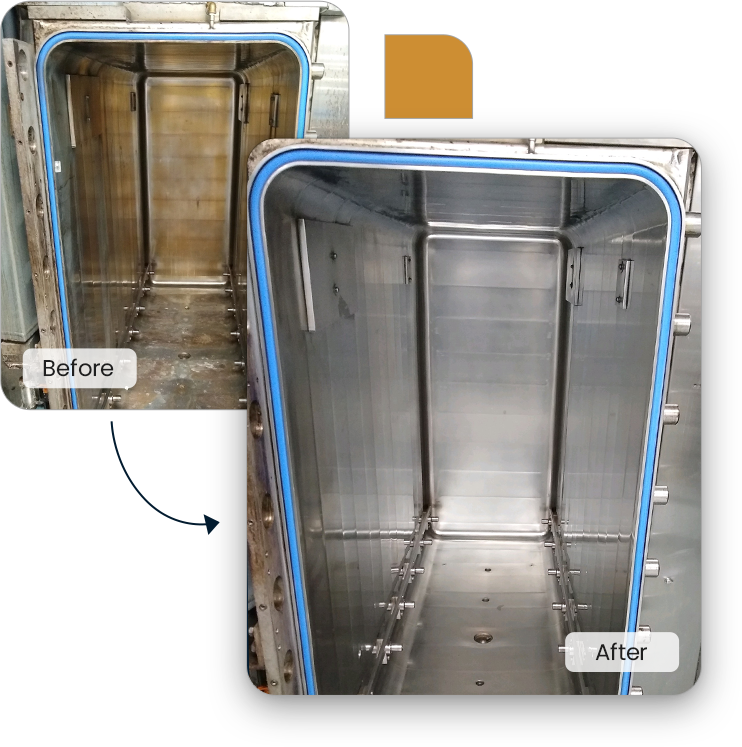
Maximizing the performance of your Sterile Processing equipment is not important…
Scheduled and effective cleaning of all your departments equipment

Credentialing organizations recommended practices
Association of periOperative Registered Nurses (AORN)
AORN 2012 periOperative Standards and Recommended Practices for Sterilization pg. 561
XV-A Policies & procedures for routine cleaning of sterilizer chambers, carts, and exterior surfaces should be developed and implemented. The sterilizer manufacturer’s written instructions for cleaning should be reviewed and followed.


Association for the Advancement of Medical Instrumentation (AAMI)
ANSI/AAMI ST79:2010 & A1:2010 & A2:2012, Section 9.4 Routine care of sterilizers
Sterilizers should be inspected and cleaned daily according to the manufacturer’s IFU
Weekly or other prescribed inspection & cleaning should be performed as specified in the manufacturer’s written IFU
Rationale: Periodic inspection & cleaning reduce the frequency of equipment malfunction and the risk of accidental contamination of sterile items